
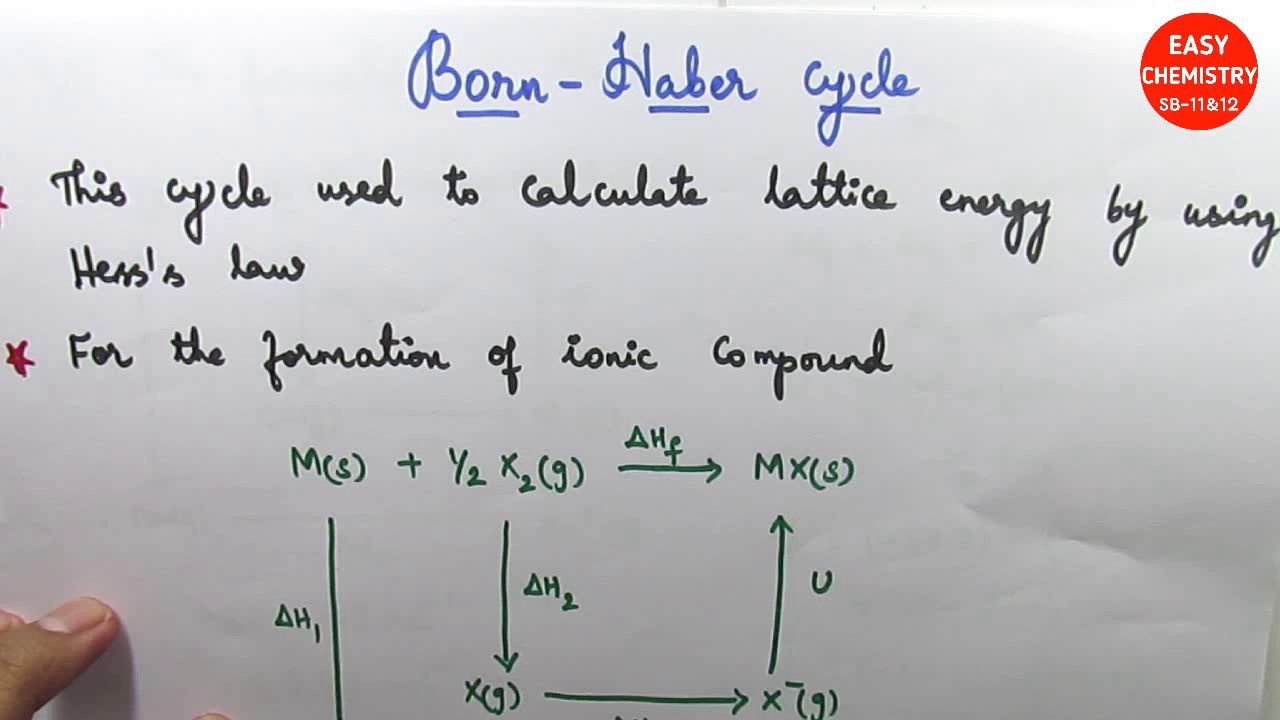
That means that we will have to use theoretical values of their lattice enthalpies. NaCl is made up of two elements sodium and chlorine in the simple ratio of 1:1. Let's look at this in terms of Born-Haber cycles of and contrast the enthalpy change of formation for the imaginary compounds MgCl and MgCl 3.
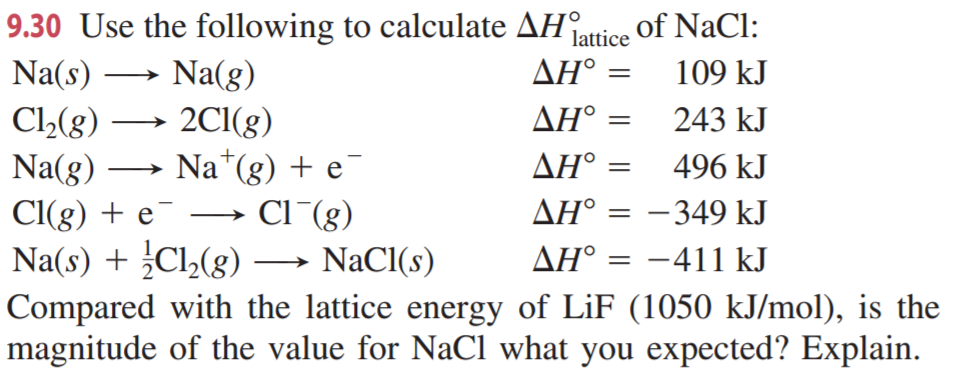
It turns out that MgCl 2 is the formula of the compound which has the most negative enthalpy change of formation - in other words, it is the most stable one relative to the elements magnesium and chlorine. The question arises as to why, from an energetics point of view, magnesium chloride is MgCl 2 rather than MgCl or MgCl 3 (or any other formula you might like to choose). Calculate the lattice energy for CaF 2.Įnergy of formation for one mole of CaF 2 from its elements = -1228 kJ/molīack to Periodic Trends and Ionic Compounds The sublimation energy for Sr is 164 kJ/mol, E i1 = +549.5 kJ/mol, E i2 = +1064.2 kJ/mol, E ea for Cl (g) = -348.6 kJ/mol, energy for the formation of one mole of SrCl 2 from its elements = -803.7 kJ/mol, and the bond dissociation energy for Cl 2 (g) = +243 kJ/mol.Įxercise 5. Which has the highest lattice energy, Na 3P or Na 2S?Įxercise 4. Order the following ionic compounds from lowest to highest lattice energy. What is the lattice energy for MgF 2?Įxercise 2. Consider the following Born-Haber cycle for MgF 2. Worksheet: Ionic Compounds, Born-Haber Cycle, and Lattice EnergyĮxercise 1. Born-Landé Equation U At r ro the potential energy must be a minimum, so U Solving for B gives U Substituting for B in the equation for the Coulombic and Born contributions to potential energy gives the Born-Landé equation,The value of n can be calculated from measurements of compressibility or estimated from theory. A more extensive list can be found in other tables or in the Handbook of Physics and Chemistry. In the case of NaCl and MgCl 2, the MgCl 2 has the larger lattice energy because the magnesium cation is smaller, and the charge is a +2 rather than a +1 for the sodium ion.īelow is a table with the lattice energies of some ionic compounds. In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. The equation for the lattice energy of NaCl is as follows First case: positive lattice energy The stability of ionic compounds is assessed using lattice energy. This is because the charges are the same, but the potassium ion is larger than the sodium ion. For example, the lattice energy of NaCl is larger than the lattice energy of KCl. Lattice energies are large when ions are closer together, the distance between the ions is small and when the charges are larger. Coulomb’s law is equal to a constant, k, multiplied by the product of the ion charges, z 1 and z 22, between the ions. Recall, lattice energy is positive meaning it is endothermic. The stronger the bond, the higher the lattice energy. Lattice energy, E lattice is dependent on the strength of the bond between the cation and anion in an ionic bond. The lattice energy is always positive, because it takes energy to separate the ions from the solid. The equation for the lattice energy is the reverse of the equation in Step 5 in the figure below, for the formation of the solid from its ions which releases 787 kJ/mol of energy.Ī Born-Haber cycle allows the calculation of the lattice energy for a solid ionic compound. The process absorbs energy, and is highly endothermic. Lattice energy, E lattice is the energy required to separate one mole of a solid ionic compound into its gaseous ions.


 0 kommentar(er)
0 kommentar(er)
