
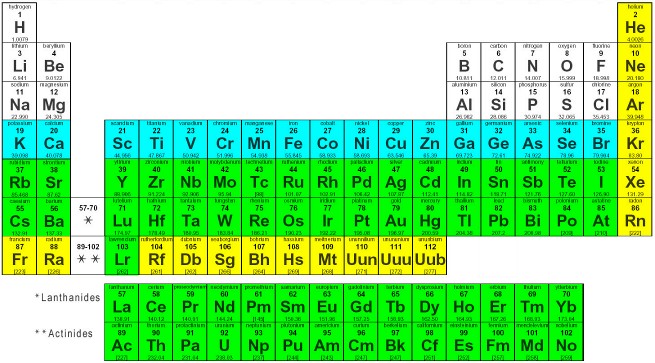
That is, group 1 elements form 1+ ions group 2 elements form 2+ ions, and so on. Moving from the far left to the right on the periodic table, main-group elements tend to form cations with a charge equal to the group number. Note the usefulness of the periodic table in predicting likely ion formation and charge (Figure 6.1b). (A discussion of the theory supporting the favored status of noble gas electron numbers reflected in these predictive rules for ion formation is provided in a later chapter.) It has the same number of electrons as atoms of the next noble gas, krypton, and is symbolized Br −.
This results in an anion with 35 protons, 36 electrons, and a 1− charge. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 17 gain one electron and form anions with a 1− charge atoms of group 16 gain two electrons and form ions with a 2− charge, and so on.

When atoms of nonmetal elements form ions, they generally gain enough electrons to give them the same number of electrons as an atom of the next noble gas in the periodic table. The name of a metal ion is the same as the name of the metal atom from which it forms, so Ca 2+ is called a calcium ion. It has the same number of electrons as atoms of the preceding noble gas, argon, and is symbolized Ca 2+. This results in a cation with 20 protons, 18 electrons, and a 2+ charge. For example, a neutral calcium atom, with 20 protons and 20 electrons, readily loses two electrons. To illustrate, an atom of an alkali metal (group 1) loses one electron and forms a cation with a 1+ charge an alkaline earth metal (group 2) loses two electrons and forms a cation with a 2+ charge, and so on. Atoms of many main-group metals lose enough electrons to leave them with the same number of electrons as an atom of the preceding noble gas. You can use the periodic table to predict whether an atom will form an anion or a cation, and you can often predict the charge of the resulting ion.
ELEMENT TABLE WITH CHARGES PLUS
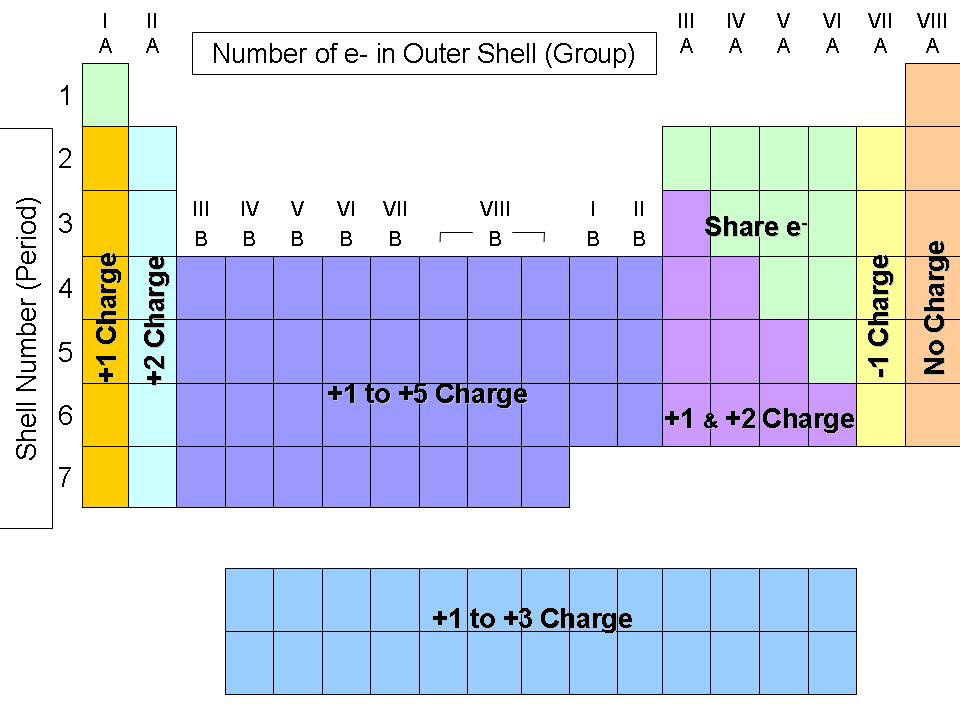
(b) A sodium cation (Na +) has lost an electron, so it has one more proton (11) than electrons (10), giving it an overall positive charge, signified by a superscripted plus sign (credit: Chemistry (OpenStax), CC BY 4.0). Figure 6.1a (a) A sodium atom (Na) has equal numbers of protons and electrons (11) and is uncharged. So keep this in mind when we're confronting different types of transition metals.By the end of this section, you will be able to:Īs a recap from Chapter 3, during the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (Figure 6.1a). They are transition metals, but they're not type two medals because they don't have multiple charges. And because of that, they're characterizes being Type two medals, some of the transition metals ones in red. Ah, lot of them have multiple possible charges. Both of them will be plus two when they do have a charge. They're both in the same group again, so they're gonna be similar to each other. So silver, when it's an ion, is gonna be plus one cadmium and zinc. But just realize here that these transition metals or called transition metals because they have a bunch of possible charges now besides the elements in Group three B or three, we also have silver, cadmium and zinc, although their transition metals as well they also have Onley one particular charge. Now, the way we're able to tell which one of these charges manganese will have will be dependent on the other element is connected to We learn about that later on. But then, of course, when you look at other transition metals, you're going to see a bunch of charges like Mangga Knees, for example, could be plus two plus three plus four plus five or even plus seven.

And there's some similar chemical properties going on for elements within that group because Skandia Miz plus three, that means the other metals that are in this group with it are also plus three. So, for example, Skandia Mom, which is in Group three or three B, it's plus three. And what we need to realize is that although many of them have multiple charges, there are quite a few that do possess only one charge. So here, if we take a look, we have some of the most common types of transition metals. So just remember, when it comes to type two medals, a majority of them are the transition metals. Now there's gonna be mawr advanced explanations for this later on, but we'll discuss them in much later chapters. Now we're gonna say most transition metals have varying positive charges because of their electron arrangements around the nucleus. So remember Type two medals are metals that possess multiple charges.


 0 kommentar(er)
0 kommentar(er)
